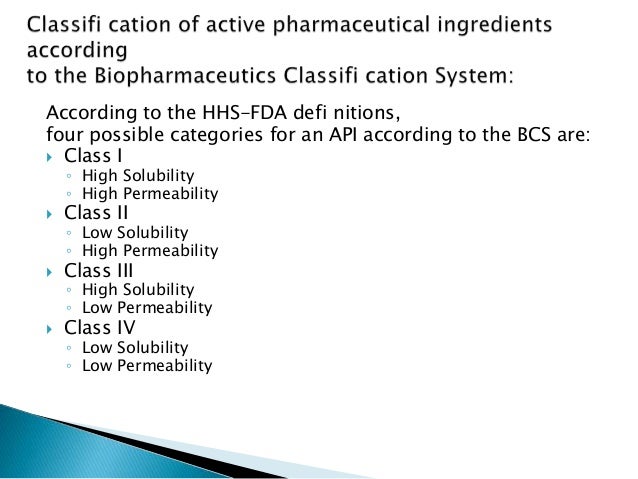
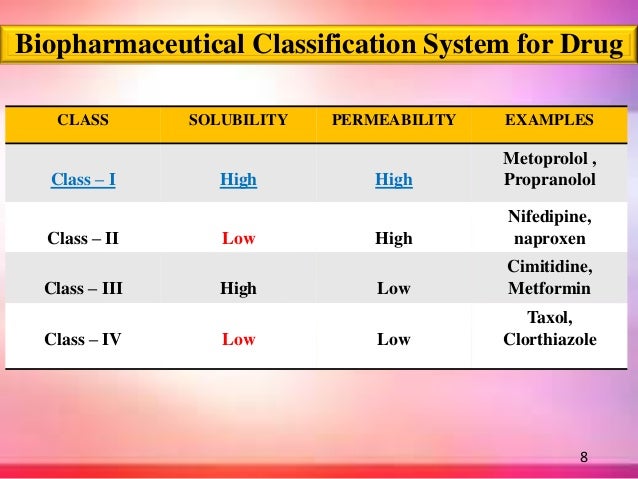
Bcs Class Iii

Bcs Classification
Class III and IV of the Biopharmaceutics Classification System (BCS) which was adopted by FDA in 2000 as a scientific basis for granting biowaivers for in vivo bioavailability and bioequivalence studies. POTENTIAL ABSORPTION BARRIERS Review has been done comprehensively to determine the barriers for the intestinal permeability of drugs.
Bcs Class 3 Drugs List Pdf
The oral route is the first envisaged route when developing a novel pharmaceutical entity since it is conducive to promoting treatment compliance. However, this route is being abandoned for numerous molecules under development on account of their low oral bioavailability. This may be due to various factors related to the very properties of the molecules or to the physiology of the gastrointestinal tract (Fasinu, et al., 2011).